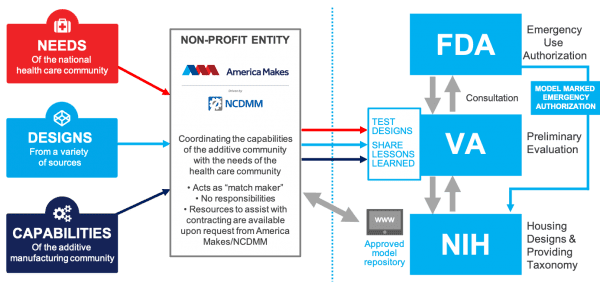
The U.S. Food and Drug Administration (FDA) is now working with the Department of Veterans’ Affairs, the National Institutes of Health (NIH), and the 3D printing trade group America Makes to provide some coherence and regulation to the work of additive companies and individuals to address the COVID-19 outbreak in the U.S.
.
The result is the COVID-19 Health Care Needs and Additive Manufacturing (AM) Capabilities Repository , an online library where AM companies can upload designs to be reviewed and placed on the NIH 3D Print Exchange. When uploaded through the America Makes submission form , 3D printable designs will be fast tracked for review. According to the organization, this approval process is meant to help determine the safety and reliability of these devices in a healthcare setting. The site will include information necessary for AM and healthcare providers that will ensure they meet medical standards for production. Through […]
New treatment that uses 3D printed implants could bring relief to knee osteoarthritis sufferers
Pioneering 'printed metal' procedure to create bespoke treatment for early knee osteoarthritis set to be trialled in...



0 Comments