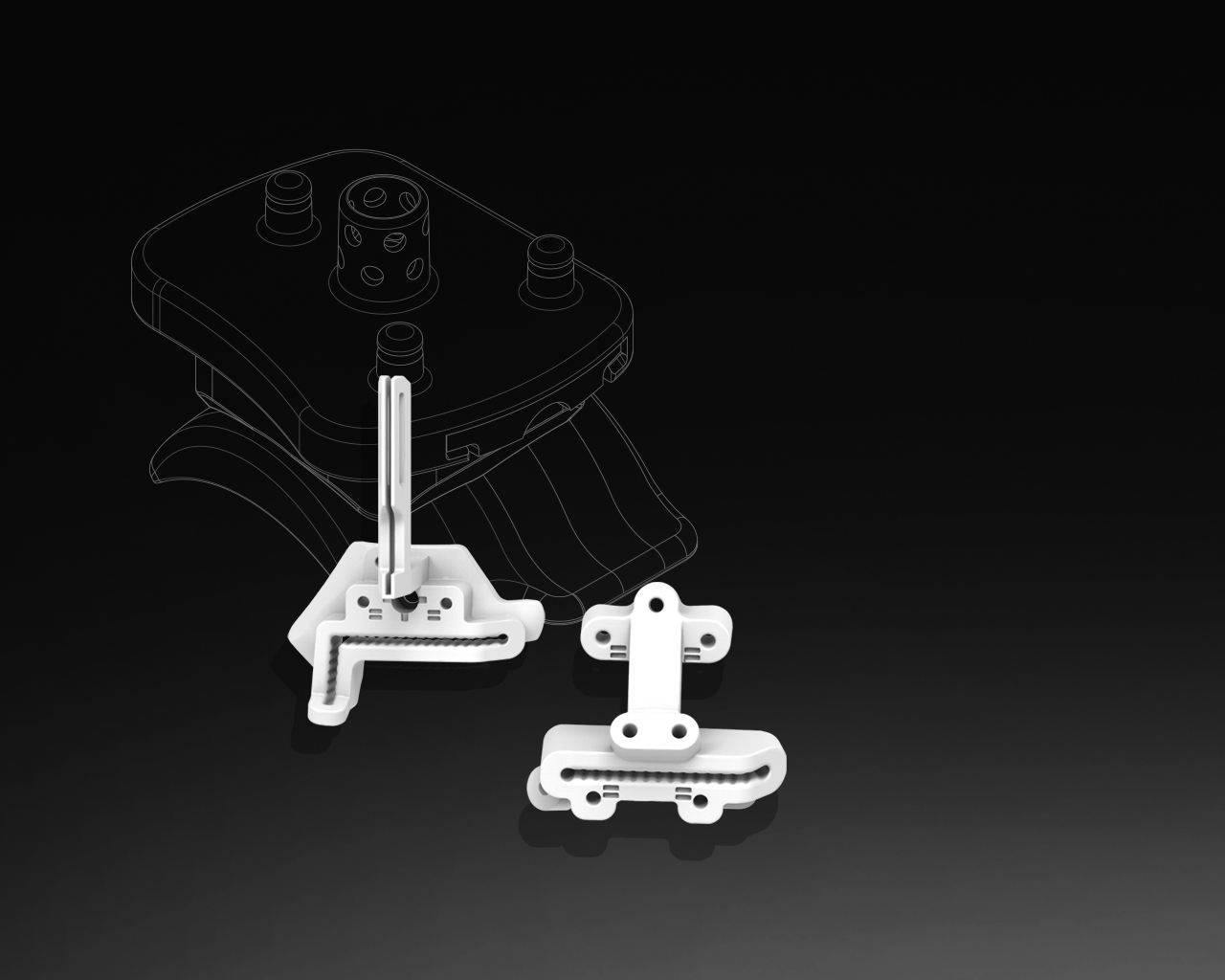
3D Systems announced the Food and Drug Administration (FDA) has provided 510(k) clearance for the Vantage Ankle PSI – its patient-specific total ankle surgical planning and 3D printed instruments. The product includes pre-surgical planning and a patient-specific 3D-printed instrument set that guides resections in the tibia and talus for total ankle replacement surgery using Exactech’s Vantage Total Ankle System.
.
Vantage Ankle PSI increases operating room efficiency, reliability, and improves soft-tissue preservation around the joint. This innovation is a result of the collaboration between 3D Systems and Exactech (Gainesville, Florida), a developer and producer of innovative implants, instrumentation, and computer-assisted technologies for joint replacement surgery. Patient-specific orthopedic instruments are an enabling technology that help surgeons prepare the skeletal anatomy to receive an implant. The Vantage Ankle PSI product, the only solution to facilitate direct patient-specific osteotomies in the ankle, is designed to increase surgical efficiency by allowing the surgeon to […]
Click here to view original web page at www.makepartsfast.com




0 Comments